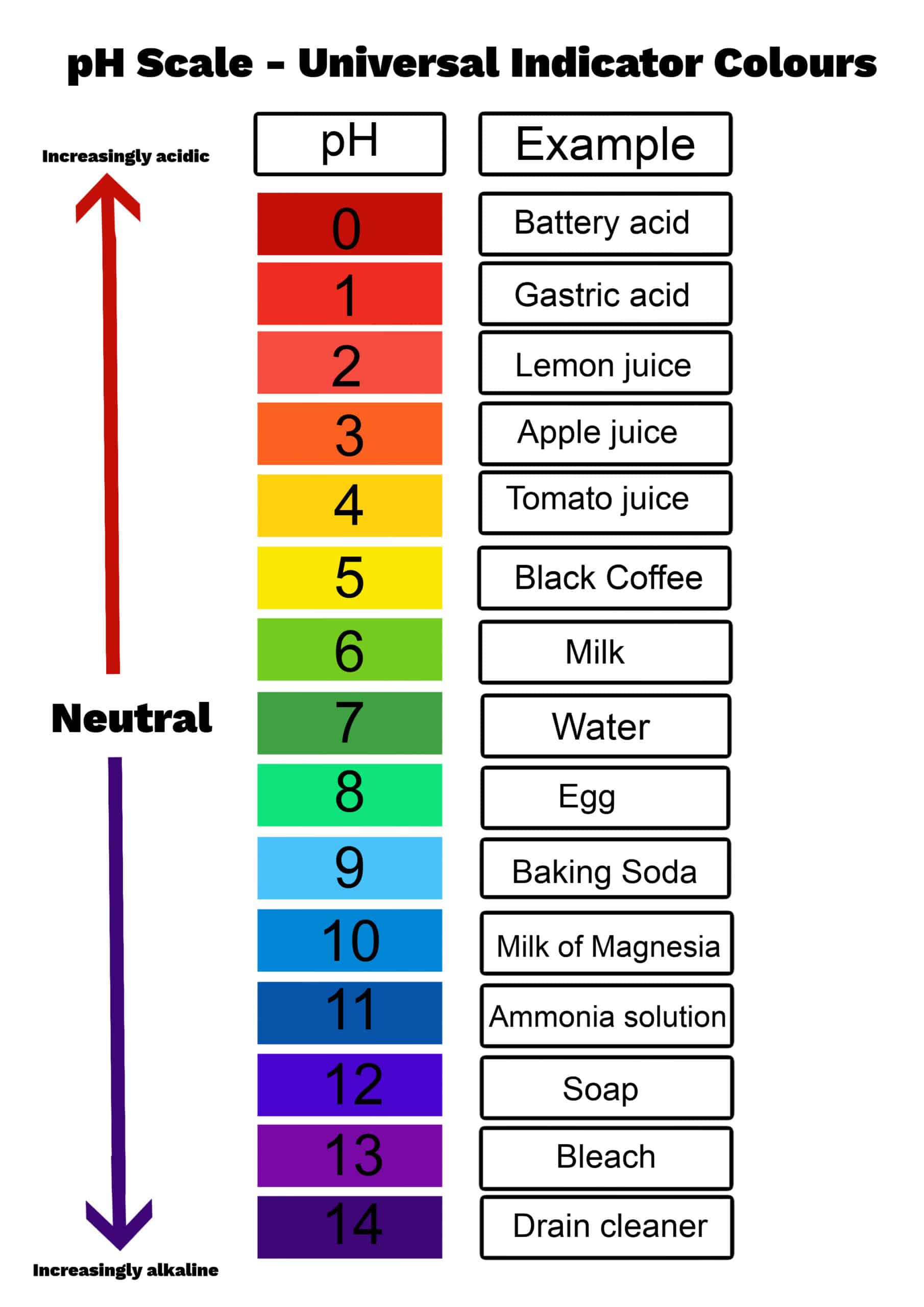
Have you ever thought about what makes a liquid sour, bitter, or just right? It turns out there's a special way we measure this, and it's called pH. This measurement tells us how acidic or how basic a liquid is, giving us a quick idea of its character. It's a bit like a secret code for liquids, letting us know if they lean one way or another on a special scale. Knowing this can be pretty helpful for all sorts of things, from making sure our drinking water is good to understanding what happens in a chemistry experiment.
This idea of pH helps us figure out if a liquid is an acid, something neutral, or a base. We use a special kind of paper, sometimes, or a neat little device to check this. The numbers on this scale usually go from zero all the way up to fourteen. If a liquid gets closer to zero, it means it's more on the acidic side, like lemon juice, for instance. If it moves closer to fourteen, that means it's more basic, like something you might use to clean. A number right in the middle, at seven, means it's neither one nor the other; it's what we call neutral, just like plain water can be.
So, when you hear about pH, it's really just a simple way to describe how a liquid behaves in terms of its sourness or bitterness. It helps us classify different watery solutions, giving us a quick snapshot of their chemical makeup. This little number, you know, gives us a lot of information about what's going on in a liquid, which is pretty neat when you think about it.
- Soywe T%C3%BCrk If%C5%9Fa
- Tsehay Hawkins Net Worth
- Mike Rowe Wife
- Good American Family
- How To Use Remote Access Mac From Raspberry Pi
Table of Contents
- What Does pH Really Mean?
- Why Is Seven So Special for Your pH Viewer?
- Does Temperature Change What Your pH Viewer Sees?
- Alkalinity and pH - Are They the Same with Your pH Viewer?
- Is It pH or pH Value - How to Write It for Your pH Viewer?
- When Things Settle - How Does Concentration Affect Your pH Viewer?
- How Does a pH Viewer Work?
- What Happens When You Water Down a Strong Base with Your pH Viewer?
- Finding the Balance Point for Molecules with Your pH Viewer
- Beyond the Usual Numbers - What Your pH Viewer Might Show in Other Liquids
What Does pH Really Mean?
So, what exactly is pH? Well, it's a way to measure how acidic or how basic a liquid happens to be. It's a kind of indicator, really, that helps us tell if a watery mix is sour like vinegar, bitter like soap, or somewhere in the middle. We use a number scale to show this. If the number is closer to zero, that liquid is very acidic. Think about, say, battery acid, which is pretty strong on that end of the scale. As the number moves up, the liquid becomes less acidic and more neutral. If it gets all the way to seven, it's perfectly neutral. Then, if it goes past seven and gets closer to fourteen, that liquid is becoming more and more basic, or alkaline, as some folks call it. For instance, things like drain cleaner are way up there on the basic side. This little number, you know, gives us a lot of information about what's going on in a liquid, which is pretty neat when you think about it.
To check this out, people often use something like litmus paper, which changes color, or a special device that gives a digital reading. This device, a kind of pH viewer, can be very precise. It helps us see where a liquid sits on that zero to fourteen spectrum. It's a simple way to classify liquids, giving us a quick idea of their chemical personality. This measurement is, in some respects, a very fundamental piece of information when you are working with any kind of solution that has water in it. It helps you, like your, understand the properties of what you are dealing with.
Why Is Seven So Special for Your pH Viewer?
When your pH viewer shows a seven, especially for water, that's a special spot. Water that reads exactly seven is considered neutral water. It's not leaning towards being acidic, and it's not leaning towards being basic either. If the reading is below seven, even just a little, that water is on the acidic side. And if the reading goes above seven, then it's on the basic side. For our bodies, and for many living things, water that is right around seven is often the best. It means the water isn't too sour and it isn't too bitter. This balance is really good for us, as a matter of fact, because our bodies function best when things are kept in a pretty stable state. So, a pH of seven is like the sweet spot for water when it comes to health and general well-being. It's a reading you often hope to see in your everyday drinking water.
- Best Remote Iot Management Software
- Poliana Arapiraca
- Hannah Taylor Influencer Age
- Bamboo Shoots Benefits
- Justin Martin Duck Dynasty Height
The reason this number seven is so important is that many biological processes, the things that happen inside living creatures, need a very specific environment to work properly. If the water is too acidic or too basic, it can mess with these processes. Think about fish in a pond, for example; they need the water to be just right, not too much acid from pollution, and not too much base either. So, when we talk about water being good for health, or good for an ecosystem, that number seven, or very close to it, is what we are usually aiming for. It's a kind of baseline, you know, for what's considered balanced and gentle. It's pretty much the ideal reading for many natural settings.
Does Temperature Change What Your pH Viewer Sees?
You might think that once you measure a liquid's pH, that's it, the number is set in stone. But actually, temperature can make a real difference in what your pH viewer shows you. When the temperature of a liquid changes, the way the pH measuring tool, like an electrode, behaves can shift quite a bit. What this means is that the reading you get might not be entirely accurate unless you account for the temperature. It's a bit like trying to measure something with a ruler that shrinks or grows depending on how hot or cold it is; you need to adjust for that change to get a true measurement. This is why, in some respects, temperature compensation is a thing when it comes to getting precise pH numbers. It helps make sure your pH viewer gives you the right information, no matter the warmth or coolness of the liquid.
So, when people talk about "pH temperature compensation," what they're really talking about is making sure the measuring device adjusts itself. It's about taking the tool's natural response to temperature and correcting it so that the reading reflects the true pH of the liquid at its current temperature. This is pretty important because if you're trying to get a very exact pH measurement, say for a science experiment or for water quality control, ignoring the temperature could lead you to some wrong conclusions. A good pH viewer will usually have a way to do this automatically, or you might need to do it manually, but the idea is to get a measurement that isn't fooled by how hot or cold the liquid is. It helps you get a truer picture of the liquid's acid or base level, which is, you know, what you're really after.
Alkalinity and pH - Are They the Same with Your pH Viewer?
It's pretty common for people to mix up alkalinity and pH, but they are actually two different ideas, even though they both relate to how a liquid behaves. Your pH viewer tells you directly how acidic or basic something is at that very moment. It's like taking a snapshot of the liquid's current sourness or bitterness. Alkalinity, on the other hand, is about how much "stuff" is in the liquid that can actually resist changes to its pH. Think of it like a buffer, a kind of protective shield. If a liquid has high alkalinity, it means it can take a lot of added acid or base before its pH number starts to shift significantly. It has a strong ability to absorb those changes without a big fuss. So, while pH is the immediate expression of sourness or bitterness, alkalinity is about the liquid's ability to keep that sourness or bitterness from changing easily. They are connected, but they describe different aspects of a liquid's properties.
For instance, if you have a pond, its pH might be neutral, say a seven. But if it has high alkalinity, that pond can handle a bit of acid rain without its pH dropping too much. The alkalinity acts like a sponge, soaking up the acid and keeping the pH stable. If the pond had low alkalinity, even a little acid rain could make its pH drop quickly, potentially harming the fish and plants. So, your pH viewer gives you the current status, but alkalinity tells you how stable that status is, how much it can take before it moves. They are, you know, both important pieces of information, but they tell you different things about a liquid's character. One is the current state, and the other is its resilience.
Is It pH or pH Value - How to Write It for Your pH Viewer?
Here's a question that pops up fairly often: should you say "pH" or "pH value"? It's a bit of a common point of confusion, and some people feel strongly about it. From what many folks remember, the proper way to write it is simply "pH," without adding the word "value" afterwards. The "H" in pH already stands for "hydrogen," and the "p" is a mathematical symbol that means "the negative logarithm of." So, "pH" itself already represents a numerical measure of hydrogen ion concentration. Adding "value" after it can seem a bit redundant, like saying "ATM machine" or "PIN number," where the last word is already part of the acronym. So, when you're writing about what your pH viewer shows, just "pH" is usually considered the more correct and streamlined way to put it. It's a small detail, but sometimes these little things matter in how we communicate scientific ideas clearly.
It's kind of like how we say "temperature" instead of "temperature value," or "weight" instead of "weight value." The term itself already implies a measurement or a quantity. While many people might use "pH value" in everyday conversation, especially if they are just getting familiar with the idea, in more formal or technical writing, sticking to just "pH" is generally preferred. It helps keep things precise and avoids unnecessary words. So, next time you are talking about the reading from your pH viewer, you know, just saying "pH" is often the way to go. It's just a little tip for clear communication.
When Things Settle - How Does Concentration Affect Your pH Viewer?
When you're dealing with liquids where things are starting to settle out, or "precipitate," the amount of stuff dissolved in the liquid, its concentration, can actually change what your pH viewer sees. For example, when a substance first begins to form solid bits and drop out of the liquid, the pH reading can be quite sensitive to how much of that substance is present. This means that if you have a very dilute liquid, the pH at which precipitation starts might be different from a very concentrated one. The initial stages of this settling process are, in some respects, quite dependent on the amount of material in the liquid. It's like the liquid is trying to figure out if it has enough dissolved material to start forming solids, and that decision point is affected by how much is there to begin with. This is, you know, a pretty interesting interaction.
However, once the settling process is complete, and everything that can possibly settle out has done so, the pH of the remaining liquid doesn't really depend on the original concentration anymore. It's as if the liquid has reached a stable point, and any extra material that was there before has now become a solid. At this stage, the pH is determined by the properties of the dissolved substances that are still in the liquid, not by how much of the original substance was present at the very beginning. So, your pH viewer would show a consistent reading regardless of the initial concentration once the precipitation is fully finished. It's a little quirk of chemistry, where the starting amount matters at first, but then it stops mattering once the reaction is complete. It's a rather neat distinction to keep in mind when you are working with solutions.
How Does a pH Viewer Work?
A pH viewer, or what many people call a pH meter, works by measuring something called "potential." This is a kind of electrical signal that develops in the liquid you are testing. Basically, the device has a special part, often a glass electrode, that creates a tiny electrical voltage when it's placed in a liquid. The amount of this voltage changes depending on how many hydrogen ions are floating around in the liquid. Since pH is all about the concentration of these hydrogen ions, the pH viewer translates that electrical signal into a pH number that you can read. It's a bit like a tiny electrical detective, sniffing out those hydrogen ions and telling you their concentration in an easy-to-understand number. This method is pretty precise, and it's why these devices are so widely used for getting accurate pH measurements. It's, you know, a very clever piece of engineering.
What's also interesting is that because a pH viewer measures this electrical potential, it can actually do more than just tell you the pH of a liquid. It can also measure the electrical force, or "electromotive force," of a battery. So, it has a couple of tricks up its sleeve, not just the one main job. The term "pH" itself has a bit of history, too. It comes from Latin, where "Pondus hydrogenii" was a phrase that meant "potential of hydrogen." This gives you a clue as to what the measurement is really getting at: the strength or activity of those hydrogen ions in a substance. So, when you look at your pH viewer, you are seeing a number that represents the hydrogen ion activity, which is, in some respects, pretty fundamental to how a liquid behaves. It's a simple number that tells a rather complex story.
What Happens When You Water Down a Strong Base with Your pH Viewer?
When you take a very strong base, something with a high pH, and you add a lot of water to it, essentially making it much weaker, the pH reading from your pH viewer will change in a specific way. If you dilute a strong base by, say, ten times, or a hundred times, or even a thousand times (which is ten to the power of 'n' times), the pH will actually go down. But here's the interesting part: it won't go down by a huge amount, and it won't go down to just any number. The diluted pH will be somewhere between its original pH minus that 'n' factor (the power of ten you diluted it by) and its original pH. It's like the pH is decreasing, but it doesn't drop as much as you might expect if you were diluting an acid. The liquid becomes less basic, but it still stays on the basic side of the scale, just not as strongly basic as it was before. It's a bit of a specific rule for strong bases, you know, when you're adding water to them. This is, actually, a pretty common thing to observe in chemistry labs.
So, for example, if you have a strong base with a pH of 13, and you dilute it by ten times (n=1), its new pH will be between 12 and 13. It won't drop all the way to 7, for instance, even with significant dilution. This is because strong bases, even when diluted, still release all their basic components into the water, just in a larger volume. The concentration of the basic ions goes down, but they are still there in full force relative to the water itself. This little rule helps you predict what your pH viewer will show you when you are mixing things up, which is pretty handy. It's a kind of guideline for understanding how these strong solutions behave when you add more liquid to them. This is, you know, a very practical piece of information for anyone working with these types of chemicals.
Finding the Balance Point for Molecules with Your pH Viewer
When we talk about things like amino acids, which are the building blocks of proteins, there's a special pH level where they become electrically balanced. This is called the "isoelectric point." Now, it's important to remember that the liquid itself always stays electrically neutral. It's the amino acid molecule within the liquid that changes its electrical charge. So, at this specific pH, the amino acid molecule itself doesn't carry a net positive or negative charge. It's like it has an equal number of positive and negative bits, so they cancel each other out. Your pH viewer helps you find this exact spot. It's the point where, if you were to look at a whole bunch of these molecules, on average, they would appear to have no overall electrical charge. This balance is really important for how proteins fold and how they work in our bodies, for instance. It's a specific condition where the molecule feels, you know, perfectly balanced in terms of its electrical properties. This is, actually, a pretty fundamental concept in biochemistry.
The isoelectric point is a unique characteristic for each type of amino acid or protein. It's like their personal electrical "sweet spot." Knowing this point is very helpful for scientists who are trying to separate or purify different proteins, because at their isoelectric point, these molecules tend to be less soluble and might even clump together. So, by carefully adjusting the pH of a liquid and watching your pH viewer, you can get molecules to reach this balanced state. This allows researchers to isolate specific proteins from a mixture. It's a very clever way to use pH to manipulate biological molecules, which is, you know, pretty cool when you think about it. It gives you a way to understand and work with these tiny building blocks of life.
Beyond the Usual Numbers - What Your pH Viewer Might Show in Other Liquids
Most of the time, when we talk about pH, we're thinking about liquids that are mostly water. And in water-based solutions, the pH scale usually stays within the zero to fourteen range. You won't see it go much higher than fourteen in water, for instance, for the same reasons we talked about with diluting strong bases. The chemistry of water just doesn't allow for much more extreme readings. However, if you step outside of water and look at other kinds of liquids, things can get pretty wild. In these "non-water systems," your pH viewer might show numbers that are much, much higher than fourteen, or even negative. For example, some types of chemicals called alkanes can have pH values that reach twenty-five or even thirty. And that's not even the highest they can go! These liquids behave very differently from water, and their acidity or basicity can be far more intense. It's a whole other ballgame when you move away from water as your main solvent. This is, you know, a very interesting area of chemistry.
And what about negative pH values? While I can't quite recall the exact numbers for how low pH can go in these non-water systems, it is possible for them to be significantly below zero. This just goes to show that the familiar zero to fourteen scale is really just for water-based liquids. When you are exploring other chemical environments, the concept of pH stretches to cover a much wider spectrum. So, if you ever come across a pH viewer reading something outside the usual zero to fourteen, especially in a non-water liquid, don't be too surprised. It's just a reminder that chemistry has many fascinating quirks beyond what we typically encounter in everyday life. It's a rather vast area of study, and these extreme pH readings are just one small part of it. It's pretty much a different way of looking at how liquids behave.
- Iot Platform Remote Control
- Delilah Distefano
- %D0%BC%D0%B0 %D1%8E%D0%B0%D0%BD%D1%8C%D0%BA%D1%83%D0%BD%D1%8C
- Is Mike Rowe Married
- Kemuri Garcia